Session 2008/2009
Fifth Report
Public Accounts Committee
Report on Delivering Pathology Services:
The PFI Laboratory and
Pharmacy Centre at Altnagelvin
Together with the Minutes of Proceedings of the committee
relating to the report and the minutes of evidence
Ordered by The Public Accounts Committee to be printed 6 November 2008
Report: 16/08/09R (Public Accounts Committee)
This document is available in a range of alternative formats.
For more information please contact the
Northern Ireland Assembly, Printed Paper Office,
Parliament Buildings, Stormont, Belfast, BT4 3XX
Tel: 028 9052 1078
Membership of Powers
The Public Accounts Committee is a Standing Committee established in accordance with Standing Orders under Section 60(3) of the Northern Ireland Act 1998. It is the statutory function of the Public Accounts Committee to consider the accounts and reports of the Comptroller and Auditor General laid before the Assembly.
The Public Accounts Committee is appointed under Assembly Standing Order No. 51 of the Standing Orders for the Northern Ireland Assembly. It has the power to send for persons, papers and records and to report from time to time. Neither the Chairperson nor Deputy Chairperson of the Committee shall be a member of the same political party as the Minister of Finance and Personnel or of any junior minister appointed to the Department of Finance and Personnel.
The Committee has 11 members including a Chairperson and Deputy Chairperson and a quorum of 5.
The membership of the Committee since 9 May 2007 has been as follows:
Mr Paul Maskey*** (Chairperson)
Mr Roy Beggs (Deputy Chairperson)
Mr Thomas Burns**
Mr Trevor Lunn
Mr Jonathan Craig
Mr Jim Wells*
Mr John Dallat
Mr Mitchel McLaughlin
Mr George Robinson****
Ms Dawn Purvis
Mr Jim Shannon*****
* Mr Mickey Brady replaced Mr Willie Clarke on 1 October 2007
* Mr Ian McCrea replaced Mr Mickey Brady on 21 January 2008
* Mr Jim Wells replaced Mr Ian McCrea on 26 May 2008
** Mr Thomas Burns replaced Mr Patsy McGlone on 4 March 2008
*** Mr Paul Maskey replaced Mr John O’Dowd on 20 May 2008
**** Mr George Robinson replaced Mr Simon Hamilton on 15 September 2008
***** Mr Jim Shannon replaced Mr David Hilditch on 15 September 2008
Table of Contents
List of abbreviations used in the Report
Report
Managing, monitoring and delivery of major capital projects
Meeting the demand for and quality of Laboratory and Pharmacy Services
The use made of exemplar design and developing specifications for projects
Appendix 1:
Appendix 2:
Appendix 3:
Correspondence of 3 November 2008 to Mr Paul Maskey, Chairperson. Public Accounts Committee
Appendix 4:
List of Abbreviations used in the Report
The Department/DHSSPS - Department of Health, Social Services and Public Safety
C&AG - Comptroller and Auditor General
DFP - Department of Finance and Personnel
NIAO - Northern Ireland Audit Office
The Trust - Western Health and Social Care Trust
The Centre - Altnagelvin Laboratory & Pharmacy Centre
Executive Summary
Introduction
1. Altnagelvin Area Hospital is part of the Western Health and Social Care Trust (the Trust) and is the largest acute hospital in Northern Ireland outside of Belfast. The development of the Laboratory and Pharmacy Services Centre (the Centre) is a key component of a £250 million redevelopment programme for the Altnagelvin Hospital complex, which is to be delivered in five phases to the end of 2015-16. The construction of the Centre, which cost £15.2 million, was funded through a public/private partnership (PFI). This will be repaid by the Trust over the 25 year term of the partnership agreement through annual payments. These will also cover the operation and maintenance of the building over the term of the Agreement. A further £3.1 million was invested by the public sector in providing a range of specialised equipment for the Centre.
2. In order to increase certainty over the cost and design of the project, the Trust made use of an “exemplar design”. This approach has been successfully used in previous procurements, such as the Belfast Cancer Centre. However, the costs of the project increased as a result of delays in progressing the project, negotiations with the preferred bidder and design changes caused, in part, by enhanced clinical accreditation standards. While it took over six years to reach contract signature, the Trust took possession of the new Centre at the end of January 2007, 10-12 weeks ahead of the schedule agreed at contract award and within the budget agreed for that contract.
3. The Committee was very impressed by the facilities, the quality and dedication of the staff and was pleased to find joined-up services operating so effectively at the Altnagelvin Hospital Complex. The Committee wishes to thank the Western Health and Social Care Trust and its staff for their hospitality and for allowing it to convene the evidence session at Altnagelvin. The Committee welcomes the opportunity to commend a complex and innovative project, funded by PFI, which overall has been well managed. Valuable lessons have been identified by both the Department and the Trust and these, together with the Committee’s recommendations, should assist public bodies in planning and managing future capital programmes and projects.
Managing, monitoring and delivery of major capital projects
4. The Committee acknowledges that managing a major capital programme involves planning and prioritising and is a highly complex process. However, it is surprised that the redevelopment of the Altnagelvin complex will not be completed until 2015-16, over 20 years after the redevelopment programme was first approved by the Department.
5. It is clear to the Committee that the route taken in settling on the PFI procurement option was circuitous and the choice between public and private sector routes should have been resolved more quickly. The Committee notes the Accounting Officer’s comments that the working assumption was that PFI would provide additional resources over and above the capital budget and as such it provided an affordability advantage. The Committee welcomes the Accounting Officer’s acknowledgement that value for money, not affordability, must be the prime consideration in deciding which procurement option should be adopted.
6. The Committee recognises that any delay in providing these new facilities would have had a negative impact on the overall strategic redevelopment programme and subsequently on patient care. The Committee also recognises that this was the first time that the Department had been involved in this type of PFI negotiation and the process proved more complex than had first been anticipated. The Committee notes the Department’s assertion that the PFI option provided certainty of both timing and budgets. However, it took over six years to deliver the Centre and this delay is reflected in the increase in unitary charges of almost £4 million over the 25 years of the contract.
7. The development of the Centre was not subject to a full Gateway Review but was subjected to a “health check”. The Committee wishes to stress the need to ensure that the Gateway review process is fully applied at the appropriate stages throughout the ongoing redevelopment at the Altnagelvin complex.
8. The Comptroller and Auditor General has previously recommended that the internal costs incurred on a project should be recorded. The Committee was disappointed to note that, despite this previous recommendation, no action had been taken by the Department or any attempt made to monitor the internal costs incurred on this project.
9. The Committee acknowledges the examples of good practice, such as the appointment of a contract-monitoring officer, and is encouraged to see examples of the Trust and its advisers being vigilant in protecting the interests of the public sector.
10. The Committee notes that the cost of advisors to this project compares favourably with other projects. Nonetheless, delays in progressing the project added to these costs. In addition, it is apparent that the Trust was over-reliant on a small number of experts in its in-house team.
Meeting the demand for and quality of Laboratory and Pharmacy Services
11. The Committee welcomes confirmation from the Department that demand for services at Altnagelvin has been broadly in line with what had been expected. However, it is concerned that, despite the impressive facilities, only three of the five laboratories have full accreditation. This is because of a shortage of consultant staff. The Committee considers that the Department must do more to ensure that there is the correct level of supply in Northern Ireland (NI) to meet demand through effective recruitment and retention practices.
12. Ideally it would have been better to have carried out a strategic review of pathology services before developing a regional facility such as that at Altnagelvin. However, the Committee notes the Department’s and Trust’s assurances that the pathology review and the Altnagelvin project were informing each other. It also notes that the Review of Public Administration has emphasised the importance of Altnagelvin’s Laboratories. The Committee welcomes the commitment of the Department to the delivery of services from the North West for the foreseeable future.
13. The Committee notes that, until recently, the Trust provided significant cytology services, funded by the Health Service Executive (HSE), to cover Counties Donegal, Sligo and Galway and that there is the potential for the satellite radiotherapy centre to provide a cross-border facility. The Trust’s location in the North West provides networking opportunities both in NI and across the border that it should seek to exploit to ensure that full use is made of the facilities at Altnagelvin.
The use made of exemplar design and developing specifications for projects
14. The Committee notes the successful use of exemplar design for the Centre, its use elsewhere, and that it has been adopted by HM Treasury as good practice. The Committee considers that the Procurement Board for Northern Ireland should explicitly consider the benefits of exemplar design to ensure that it is given sufficient weight when any major projects are being considered.
15. The Department acknowledges that technological advances are difficult to foresee. In this context, the Committee is surprised that the public sector took on the responsibility and associated risk for the procurement of equipment, which will most likely develop and change over the next 25 years. The Committee considers that more effort should have been put into building a detailed specification for bidders of equipment requirements.
Summary of Recommendations
Managing, monitoring and delivery of major capital projects
1. It is important in preparing their investment plans that Departments show a clear link between programmes or projects and their contribution towards priority outputs and outcomes. In that context, investment plans must include indicative costs and timetables for delivery, thereby increasing clarity and accountability.
2. The Committee recommends that public bodies must make decisions regarding the proposed funding arrangements for major capital programmes and projects early in the procurement process and again before a preferred bidder is selected, when the basis of a deal should be clear. In making a decision on the procurement route to be adopted, value for money must be the prime consideration.
3. The Committee recommends that the Department should apply the Gateway review process fully to the remaining programme of redevelopment at the Altnagelvin Complex. All Departments and public bodies must ensure that the Gateway process is used at the appropriate stages on all major capital programmes and projects.
4. The Committee is concerned at Departments ignoring recommendations contained in reports produced by the Comptroller and Auditor General. It is important that Departments respond quickly and appropriately to these. In relation to monitoring internal costs, the Committee recommends that this should be done for all major projects. This should involve setting realistic budgets at the outset, monitoring these and ensuring they are kept under control.
5. Public bodies need to be aware of the risks of placing reliance on a small number of individuals in a project team. The Committee recommends that major capital projects should always be fully supported by a properly costed and appropriately skilled internal project management structure.
Meeting the demand for and quality of Laboratory and Pharmacy Services
6. The Committee has previously raised concerns surrounding accreditation levels and the difficulties in recruiting consultants to pathology at Altnagelvin. It is important that the Department continues to improve the level of accreditation to meet its published targets for accreditation. This must include the effective planning and managing of an expert workforce and ensuring that there is the correct level of supply to meet demand through recruitment and retention practices.
7. The Committee recommends that, to make the most effective use of the new facilities, the Department and Trust should seek to maximise networking opportunities to provide such services outside the Trust’s area in Northern Ireland, Donegal and the other border counties.
The use made of exemplar design and developing specifications for projects
8. Whilst the Committee recognises the benefits of applying an exemplar design approach, it is imperative that it is applied in a timely way and that changes to the design are managed so as to minimise increases in costs.
9. The Committee recommends that the Procurement Board for NI should explicitly consider the benefits of exemplar design to ensure that it is given sufficient weight when any major projects are being considered.
10. The Committee recommends that, in order to achieve value for money, public sector bodies must clearly define their required output specifications; this should include greater dialogue with potential bidders about what is required, before approaching the market.
Introduction
1. The Public Accounts Committee met on 2 October 2008 to consider the Comptroller and Auditor General’s report: “Delivering Pathology Services: The PFI Laboratory and Pharmacy Centre at Altnagelvin” (NIA 9/08-09, Session 2008-09). The witnesses were:
- Dr Andrew McCormick, Permanent Secretary of the Department of Health, Social Services and Public Safety
- Mrs Elaine Way, Chief Executive, Western Health and Social Care Trust
- Dr Jim Livingstone, Director of Safety, Quality and Standards in the Department of Health, Social Services and Public Safety
- Mr John Dowdall CB, Comptroller and Auditor General
- Mr David Thomson, Treasury Officer of Accounts, Department of Finance and Personnel
2. The Committee was very impressed by the facilities, the quality and dedication of the staff and was pleased to find that joined-up services were operating so effectively at the Altnagelvin Hospital Complex. The Committee wishes to thank the Western Health and Social Care Trust and its staff for their hospitality, presentation and tour of the new facilities at the Laboratory and Pharmacy Services Centre and is also grateful to the Trust for allowing it to convene the evidence session at Altnagelvin.
3. Altnagelvin Area Hospital is part of the Western Health and Social Care Trust (the Trust) and is the largest acute hospital in Northern Ireland outside of Belfast. The development of the Laboratory and Pharmacy Services Centre (the Centre) is a key component of a £250 million redevelopment programme for the Altnagelvin Hospital complex, which is to be delivered in five phases to the end of 2015-16. The construction of the Centre, which cost £15.2 million, was funded through a public/private partnership (PFI). This will be repaid by the Trust over the 25 year term of the partnership Agreement through annual payments of £1.6 million; these payments will also cover the operation and maintenance of the building over the term of the Agreement. A further £3.1 million was also invested by the public sector in providing a range of specialised equipment for the Centre.
4. In order to increase certainty over the cost and design of the project, the Trust made use of an “exemplar design”. This approach has been successfully used in previous procurements, such as the Belfast Cancer Centre. However, the costs of the project increased as a result of delays in progressing the project, negotiations with the preferred bidder and design changes caused, in part, by enhanced clinical accreditation standards. While it took over six years from the appointment of advisors to contract signature in April 2005, the Trust took possession of the new Centre at the end of January 2007, 10-12 weeks ahead of the schedule agreed at contract award and within the budget agreed for that contract. The Committee welcomes the opportunity to commend a complex and innovative project, funded by PFI, which overall has been well managed. Valuable lessons have been identified by both the Department and the Trust and these, together with the Committee’s recommendations, should assist public bodies in planning and managing their capital programmes and projects.
5. In taking evidence, the Committee focussed on the following issues;
- Managing, monitoring and delivery of major capital projects
- On meeting the demand for and quality of laboratory and pharmacy services
- The use made of exemplar design and developing specifications for projects
Managing, Monitoring and Delivery of Major Capital Projects
6. The Committee acknowledges that managing a major capital programme involves planning and prioritising and is a highly complex process. However, the Committee was surprised that the redevelopment of the Altnagelvin complex will not be completed until 2015-16, over 20 years after the redevelopment programme was first approved by the Department. This is much too long, particularly in the context of a hospital which, as the largest acute hospital outside of Belfast, is seeking to secure sustainable, safe and high-quality services for patients and clients in the region.
7. The Committee recognises that this was a complex and innovative project, funded by PFI, which has been well managed overall. However, it is clear to the Committee that the route taken in settling on the PFI procurement option was circuitous and the choice between public and private sector routes should have been resolved more quickly. The Committee notes the Accounting Officer’s comments that the working assumption was that PFI would provide additional resources over and above the capital budget set by the Department of Finance and Personnel (DFP) and as such it provided an affordability advantage. The Committee welcomes the Accounting Officer’s acknowledgement that value for money, not affordability, must be the prime consideration in deciding which procurement option should be adopted.
Recommendation 1
8. It is important in preparing their investment plans that Departments show a clear link between programmes or projects and their contribution towards priority outputs and outcomes. In that context, the Committee recommends that plans must include indicative costs and timetables for delivery, thereby increasing clarity and accountability.
Recommendation 2
9. The Committee recommends that public bodies must make decisions regarding the proposed funding arrangements for major capital programmes and projects early in the procurement process and again before a preferred bidder is selected, when the basis of a deal should be clear. In making a decision on the procurement route to be adopted, value for money must be the prime consideration.
10. The Committee recognises that any delay in providing these new facilities would have had a negative impact on the overall strategic redevelopment and subsequently on patient care. The Committee also notes the Department’s assertion that the PFI option provided certainty of both timing and budgets. However, it took over six years to deliver the Centre. Those delays, caused by confusion over the source of funding; changes to the exemplar design, due in part to enhanced clinical accreditation standards; and negotiations with the preferred bidder, led to significant increases in costs. This is reflected in the increase in unitary charges of almost £4 million over the 25 years of the contract.
11. The Committee acknowledges that this was the first time that the Department had been involved in this type of PFI negotiation and the process had proved more complex than had first been anticipated. However, it is clear that the delivery of the Centre took longer than was hoped for and that there is clearly room for improving such an outcome. The Committee notes that HM Treasury has found that similar, lower value PFI capital projects have also faced difficulties, including disproportionately large procurement times. The Committee considers that this was evident in this project.
12. While there was no requirement to carry out a full Gateway Review as the project was well advanced when this became compulsory, the Trust arranged for an independent “health check”. The Committee commends the Trust for taking this action and on the positive outcome of that review. However, the Committee wishes to stress the need to ensure that the Gateway review process is fully applied at the appropriate stages throughout the ongoing redevelopment of the Altnagelvin complex.
Recommendation 3
13. The Committee recommends that the Department should apply the Gateway review process fully to the remaining programme of redevelopment at the Altnagelvin Complex. All departments and public bodies must ensure that the Gateway process is used at the appropriate stages on all major capital programmes and projects.
14. In a 2004 NIAO Report on the Funding and Management of three PFI projects in the Health Sector, the Comptroller and Auditor General recommended that the internal costs on a project should be recorded. The Committee was disappointed to note that; despite this previous recommendation, no action had been taken by the Department, or any attempt made to monitor the internal costs on this project. The Department needs to be much more responsive in taking such recommendations on board in future.
Recommendation 4
15. The Committee is concerned at Departments ignoring recommendations contained in reports produced by the Comptroller and Auditor General. It is important that Departments respond quickly and appropriately to these. In relation to monitoring internal costs, the Committee recommends that this should be done for all major projects. This should involve setting realistic budgets at the outset, monitoring these and ensuring they are kept under control.
16. The Committee commends the dedication of the staff at Altnagelvin and examples of good practice, such as the appointment of a contract-monitoring officer, who is tasked with keeping a close eye on the contract to ensure that any changes that arise are well managed.
17. The Committee was encouraged to see the Trust and its advisers being vigilant in protecting the interests of the public sector, for example in identifying and negotiating a share of windfall profits. The Committee notes that the cost of advisors to this project compares favourably with other projects. However, delays in progressing the project added to these costs. In addition, it was acknowledged by the Trust that it had been over-reliant on, and therefore placed undue pressure on, a small number of experts in their in-house team.
Recommendation 5
18. Public bodies need to be aware of the risks of placing reliance on a small number of individuals in a project team. The Committee recommends that major capital projects should always be fully supported by a properly costed and appropriately skilled internal project management structure.
Meeting the Demand for and Quality of Laboratory and Pharmacy Services
19. The Committee was pleased to learn that demand for services at Altnagelvin has been broadly in line with what had been expected. However, the Committee is concerned that, despite the impressive facilities at the Centre, only three of the five laboratories have full accreditation, because of a shortage of consultant staff. The Committee previously considered the issue of accreditation in 2002 and was concerned that in June 2000 only 63 per cent of pathology services in NI had either full or partial accreditation. The Committee notes that the comparable level of accreditation currently stands at 79 per cent and that the Department’s recommendations for the future of pathology services, announced in December 2007, commits it to achieving full accreditation by 2010.
20. The Committee recognises the shortage of pathologists across the UK. It welcomes the positive feedback that the Trust has received from potential candidates, who have been impressed by both the working environment and the team at Altnagelvin. The Committee also recognises the Trust’s commitment to filling vacancies which is demonstrated by the relationship with the universities and other training institutions. The Committee is reassured by the Department’s assertion that despite staffing problems they are delivering a safe and effective level of service across Northern Ireland. However, the Committee considers that the Department must do more to ensure that there is the correct level of supply to meet demand through effective recruitment and retention practices.
Recommendation 6
21. The Committee has previously raised concerns surrounding accreditation levels and the difficulties in recruiting consultants to pathology at Altnagelvin[1]. The Committee recommends that the Department continues to improve the level of accreditation to meet its published targets for accreditation. This must include the effective planning and managing of an expert workforce and ensuring that there is the correct level of supply to meet demand through recruitment and retention practices.
22. Ideally it would have been better to have carried out a strategic review of pathology services before developing a regional facility such as that at Altnagelvin. However, the Committee takes comfort from the Department’s and Trust’s assurances that the pathology review and the Altnagelvin project were informing each other. It also notes that the Review of Public Administration has emphasised the importance of Altnagelvin’s laboratories. The Committee also welcomes the commitment of the Department to the delivery of services from the North West for the foreseeable future.
23. The Committee is disappointed at the Department’s inability to provide current costs of individual pathology procedures in the new Laboratory at Altnagelvin, relative to the costs of the same procedures at other Laboratories in NI at this time. This would have helped the Committee to gain a clearer picture of the benefits being delivered by the Centre. The Committee looks forward to receiving this information together with the Department’s analysis in due course.
24. The Committee notes with interest that, until recently, the Trust provided significant cytology services, funded by the Health Service Executive (HSE), to cover Counties Donegal, Sligo and Galway and that there is the potential for the satellite radiotherapy centre to provide a cross-border facility. The Trust’s location in the North West provides networking opportunities both in NI and across the border. In the Committee’s view, it is important that the Department and the Trust maximise opportunities to make full use of the new facilities at Altnagelvin.
Recommendation 7
25. The Committee recommends that, to make the most effective use of the new facilities the Department and Trust should seek to maximise networking opportunities to provide such services outside the Trust’s area in Northern Ireland, Donegal and the other border counties.
The use made of Exemplar Design and Developing Specifications for Projects
26. The Committee notes the successful use of exemplar design for the Centre, its use elsewhere and that it has been adopted by HM Treasury as good practice. The Committee was told by the Treasury Officer of Accounts that there have been other examples of exemplar design and although DFP does not promote exemplar design as such, its usage is not precluded by the “Achieving Excellence in Construction” guidance.
Recommendation 8
27. Whilst the Committee recognises the benefits of applying an exemplar design approach, it recommends that it is applied in a timely way and that changes to the design are managed so as to minimise increases in costs.
Recommendation 9
28. The Committee recommends that the Procurement Board for Northern Ireland should explicitly consider the benefits of exemplar design to ensure that it is given sufficient weight when any major projects are being considered.
29. The Department acknowledges that technological advances are difficult to foresee. In that context, the Committee is surprised that the public sector took on the responsibility and associated risk for the procurement of equipment, which will most likely develop and change over the next 25 years; in contrast to the approach adopted in the Belfast Cancer Centre. The Committee acknowledges that the assessment of the proposed bids for the procurement of equipment identified major concerns and would have made the service unsafe. The Committee is reassured by the Accounting Officer’s comments that all the equipment which was procured for the Centre has gone through the proper analytical and approval process to ensure that value for money has been secured. However, the Committee questions why the Trust provided a list of equipment that was already in use, rather than putting more effort into building a detailed specification for bidders of equipment requirements.
Recommendation 10
30. The Committee recommends that, in order to achieve value for money, public sector bodies must clearly define their required output specifications; this should include greater dialogue with potential bidders about the design of assets, before approaching the market.
[1] A Review of Pathology Laboratories in Northern Ireland, Sixth Report, Session 2001/2002, 20 February 2002.
Appendix 1
Minutes of Proceedings of the Committee Relating to the Report
Thursday, 2 October 2008
The Denis Desmond Room,
Western Health and Social Care Trust Headquarters, Altnagelvin Hospital
Present: Mr Paul Maskey (Chairperson)
Mr Roy Beggs (Deputy Chairperson)
Mr Jonathan Craig
Mr John Dallat
Mr Trevor Lunn
Mr Mitchel McLaughlin
Ms Dawn Purvis
Mr George Robinson
Mr Jim Shannon
Mr Jim Wells
In Attendance: Mr Jim Beatty (Assembly Clerk)
Ms Alison Ross (Assembly Clerk)
Mrs Gillian Lewis (Assistant Assembly Clerk)
Mr John Lunny (Clerical Supervisor)
Apologies: None
The meeting opened at 2.00pm in public session.
1. Apologies.
None.
The Chairperson welcomed Mr John Dowdall CB, Comptroller and Auditor General (C&AG) and Mr David Thomson, Treasury Officer of Accounts (TOA) to the meeting.
2. Matters arising
Members considered correspondence between the Clerk and the Clerk to the Committee of Finance and Personnel (CFP).
2.06pm Mr Dallat joined the meeting.
Agreed: Members agreed that the Clerk would inform the Clerk to the CFP that issues under consideration by the Public Accounts Committee (PAC) should not be scrutinised by the CFP until the PAC has published its report and considered the Memorandum of Reply in relation to the report. However, members agreed that CFP should continue with scrutiny of any other matters which fall outside the scope of the NIAO report, and the PAC’s subsequent investigations.
3. Evidence on the NIAO Report ‘Delivering Pathology Services: The PFI Laboratory and Pharmacy Centre at Altnagelvin’.
The Committee took oral evidence on the NIAO report ‘Delivering Pathology Services: The PFI Laboratory and Pharmacy Centre at Altnagelvin’ from Dr Andrew McCormick, Accounting Officer, Department of Health, Social Services and Public Safety (DHSSPS), Mr Jim Livingstone, Director of Safety, Quality and Standards, DHSSPS, and Mrs Elaine Way, Chief Executive, Western Health and Social Care Trust.
The witnesses answered a number of questions put by the Committee.
Members requested that the witnesses provide additional information to the Clerk on some issues raised as a result of the evidence session.
3.46pm The evidence session finished and the witnesses left the meeting.
[EXTRACT]
Thursday, 6 November 2008
Room 144, Parliament Buildings
Present: Mr Paul Maskey (Chairperson)
Mr Roy Beggs (Deputy Chairperson)
Mr Thomas Burns
Mr Jonathan Craig
Mr John Dallat
Mr Trevor Lunn
Mr Mitchel McLaughlin
Ms Dawn Purvis
Mr George Robinson
Mr Jim Shannon
Mr Jim Wells
In Attendance: Mr Jim Beatty (Assembly Clerk)
Ms Alison Ross (Assembly Clerk)
Mr John Lunny (Clerical Supervisor)
Mr Darren Weir (Clerical Officer)
The meeting opened at 2.01pm in public session.
3. Apologies.
None.
4. Minutes of the meeting on 23 October 2008.
Agreed: The minutes were agreed.
5. Matters arising.
Members noted that correspondence to Accounting Officers had been issued as agreed.
2.05pm Mr Craig joined the meeting.
6. Forward Work Programme.
Members discussed the draft forward work programme for the period January to April 2009.
Agreed: Members agreed the forward work programme for the period January to April 2009
7. Assembly Commission Report.
Members considered the draft Committee entry for the Assembly Commission Report.
Agreed: Members agreed the entry without amendments.
8. Retirement of the C&AG
Members noted that the C&AG will retire in September 2009 and that the appointment of his replacement is being taken forward by the Director-General’s office.
2.10 Mr Dallat joined the meeting.
9. NIAO Draft Corporate Plan
Mr John Dowdall, C&AG; Mr Kieran Donnelly, Deputy C&AG and Louise Mason, AAG spoke to the Draft Corporate Plan and answered Member’s questions.
10. Briefing on the NIAO Report: ‘Shared Services for Efficiency – A Progress Report’
Mr John Dowdall, C&AG; Mr Brandon McMaster, Director of Value for Money; Mr Sean Beattie, Audit Manager, and Mr Joe Campbell, Audit Manager, briefed the Committee on the NIAO Report, ‘Shared Services for Efficiency – A Progress Report’, and answered members’ questions.
2.36pm The meeting went into closed session.
2.50pm Mr Burns left the meeting.
11. Issues from Oral Evidence Session on NIAO Report: ‘Warm Homes: Tackling Fuel Poverty’
Members considered the Issues Paper.
Agreed: Members agreed the Issues Paper.
12. Consideration of the Committee’s Report ‘Delivering Pathology Services: The PFI Laboratory and Pharmacy Centre at Altnagelvin’
Members considered the draft report paragraph by paragraph.
The Committee considered the main body of the report.
2.59pm Mr Burns returned to the meeting
Paragraphs 1 – 3 read and agreed.
Paragraphs 4 - read, amended and agreed.
Paragraphs 5 - read and agreed.
Paragraph 6 - read, amended and agreed.
Paragraphs 7 - 13 read and agreed.
Paragraph 14 - read, amended and agreed.
Paragraphs 15 – 17 read and agreed.
Paragraph 18 – read, amended and agreed.
Paragraph 19 – read and agreed.
Paragraphs 20 – 21 read, amended and agreed.
Paragraph 22 – read and agreed.
Paragraphs 23 – 24 read, amended and agreed.
Paragraph 25 – 26 read and agreed.
Paragraph 27 – 29 read, amended and agreed.
Paragraph 30 – read and agreed
3.12pm Mr Lunn left the meeting.
The Committee considered the Executive Summary.
Paragraphs 1 - read and agreed.
Paragraph 2 - read, amended and agreed.
Paragraph 3 - read and agreed.
Paragraphs 4 - read, amended and agreed.
Paragraphs 5 – 7 read and agreed.
Paragraphs 8 - read, amended and agreed.
Paragraphs 9 – 10 read and agreed.
Paragraph 11 read, amended and agreed.
Paragraph 12 - 14 read and agreed.
Paragraphs 15 - read, amended and agreed.
Agreed: Members ordered the report to be printed.
Agreed: Members agreed that the Chairperson’s letter to the Accounting Officer, Department of Health, Social Services & Public Safety, requesting further information, and the Accounting Officer’s response should be included in the report.
Agreed: Members agreed that the Post Project Evaluation Report would be laid in the Assembly Library and published on the Committee website.
Agreed: Members agreed an embargo date of 00h01 on 27 November 2008 for the report.
Agreed: It was agreed that the Clerk would take the issue of publicity surrounding the Report forward with the Assembly’s Press Officer.
[EXTRACT]
Appendix 2
Minutes of Evidence
2 October 2008
Members present for all or part of the proceedings:
Mr Paul Maskey (Chairperson)
Mr Roy Beggs (Deputy Chairperson)
Mr Jonathan Craig
Mr John Dallat
Mr Trevor Lunn
Mr Mitchel McLaughlin
Ms Dawn Purvis
Mr George Robinson
Mr Jim Shannon
Mr Jim Wells
Witnesses:
| Dr Jim Livingstone Dr Andrew McCormick |
Department of Health, Social Services and Public Safety | |
| Mrs Elaine Way | Western Health and Social Care Trust |
Also in Attendance:
| Mr John Dowdall CB | Comptroller and Auditor General | |
| Mr David Thomson | Treasury Officer of Accounts |
1. The Chairperson (Mr P Maskey): The Committee is considering the Comptroller and Auditor General’s report ‘Delivering Pathology Services: The PFI Laboratory and Pharmacy Centre at Altnagelvin’. I welcome Dr Andrew McCormick, accounting officer for the Department of Health, Social Services and Public Safety (DHSSPS), Dr Jim Livingstone, director of safety, quality and standards for the DHSSPS, and Mrs Elaine Way, chief executive of the Western Health and Social Care Trust.
2. Thank you for allowing the Committee to visit the laboratories today. Several members were unable to visit as they had made previous arrangements. However, those who participated found the tour interesting. Please thank the staff who took time out from their busy schedules to show us around.
3. Members of the Committee will ask questions, and I, as Chairperson, have the privilege of asking the first question. Paragraph 1.2 of the report sets out the funds that were allocated for the Altnagelvin complex in the context of delivering the North’s investment strategy. The centre was funded by a public-private partnership, and the equipment was procured by the Department of Health, Social Services and Public Safety. How does the Department prioritise major capital projects, how will they be funded, and how is delivery monitored and reported?
4. Dr Andrew McCormick (Department of Health, Social Services and Public Safety): Thank you for giving us the opportunity to give a presentation on this important topic. Given that managing a major capital programme involves planning and prioritising and is a highly complex process, it is worth my putting the timing of the project in context.
5. The Department worked on the investment strategy from 2002 to 2005, during which time certain decisions were made. Our approach in prioritising projects is to consider a range of highly complex and highly diverse needs. The Department manages a system that covers all aspects of health and social care, and it must have regard to its responsibilities on fire-and-rescue services. The capital allocation at the early part of this decade was running at around £80 million a year, and the Department needed to ascertain what were the most important projects.
6. The criteria that were used included examining the fundamental need for a project, taking into consideration the services that would need to be provided; the impact on patient care; addressing the health-and-safety issues that arise in many of the facilities that the Department operates; and securing the best possible outcomes for patients and clients. The full range of health-and-social care as well as fire-and-rescue services must be covered. The Department must identify and recognise any major risks or demands as well as changes in the use of facilities. A vital part of the planning involves considering the overarching acute hospital strategy — Developing Better Services — to ensure that there is a managed and effective change process to secure sustainable and safe high-quality services for the region.
7. Acute hospitals are the largest and most expensive of the facilities that the Department manages. However, if the acute sector were the only area with which the Department dealt, it would miss out on funding the major needs of social care, mental health, ambulances and fire and rescue. For example, an announcement was made yesterday about ambulance services. Ensuring that there is a fair and equitable geographic spread of projects, which must be examined to ensure that they are handled properly, is also a major issue for the Department.
8. Therefore, the process is highly complex, and it must then be built into a strategy. The Department must ensure that it adopts the right match of funding flow to a planned programme of delivery.
9. We must identify the resources that are available and decide how best to phase and manage them. When this project was being planned and developed, the working assumption was that a PFI would provide additional resources over and above the capital budget set by the Department of Finance and Personnel (DFP). It would be funded from the revenue budget instead, because we would be paying a unitary charge to a developer over 25 years rather making an upfront capital payment.
10. That was the assumption when the decision was made. We were trying to secure the best use of all the resources that were available in order to ensure a totality of outcome for the region; that is, the right hospitals and the right provision of all aspects of the services for which we were responsible. After that, to answer the second part of your question, monitoring and managing those resources involved making sure that projects were managed effectively by each of the responsible organisations.
11. At that time, there were 18 health and social care trusts and the Ambulance Service Trust, meaning that there was quite a complex administrative structure. However, each organisation had to take responsibility themselves for delivering the projects. Therefore, the Department’s role was to work with each delivery organisation to provide, through health estates, a centre of procurement excellence. They exist to ensure that procedures are well managed and that the project managers are doing the right things in order to deliver value for money and fit-for-purpose outcomes.
12. I hope that I have put in context the processes with which we must work and provided some background on what we did on the project.
13. The Chairperson: Thank you very much, Dr McCormick. Paragraph 1.3 of the Audit Office report states that the trust saw the completion of phase 2 of the Altnagelvin Hospital redevelopment as:
“critical to the successful delivery of the overall Altnagelvin redevelopment programme.”
If that was the case, why did it take over six years to deliver the centre?
14. Dr McCormick: The project took longer than was hoped for at the time. As the report makes clear, it was hoped that things would move more quickly. However, it is important to set that in the context of the nature of the procurement process in which we were involved and the need to ensure that the right decisions were made at each step. This was the first time that that particular procurement model was used, certainly by the trust, and even the Department had never been involved in that type of PFI negotiation before.
15. The negotiated procedure that had been set down in the procurement regulations no longer exists. It has been overtaken, partly because it was decided at UK and European level that a feature of that negotiated process was a tendency towards avoidable delay, and some extension of process, that had to be sharpened up. That lesson — to improve processes — was learned across Europe, but this was the first occasion on which the new procedure was used here.
16. The negotiated procedure allows for the various stages, and those are set out in the report. It means that alongside the first key stage of the process — the invitation to bidders to negotiate — there is a protracted process in which the project is being developed by an iterative negotiation. At the previous stage, before invitations to negotiate can be issued, we have to be very sure of our ground in planning a project by that process, given that the contract was to be for 25 years. That means being able to see round corners and determine what might happen, allow for risks, and ensure that the definition of risk and who might carry such risk is set out formally and legally.
17. That meant that the legal advisers had a great deal of work to do. The process proved more complex than had first been anticipated, and it took longer than anyone would have hoped for at the start. However, at each step of the way, the management team sought to make the right decisions.
18. The team was working through an evolving situation. As the process unfolded, changes were taking place in building standards and in clinical standards, and at each stage the team had to adapt to those changes. That took time. Occasionally, an opportunity to do something better arose, and that meant choosing between taking the time to make those improvements or rushing ahead with the project to save time. In the past, people have been criticised for making the wrong decision under time pressure, and the team had to make a series of balanced judgements as to what was the right thing to do week in, week out and month in, month out. The consequence was the sequence of events that unfolded. There is clearly room for improving such an outcome, but the team was legitimately making genuine, honest decisions about the best thing to do at each stage.
19. Mrs Elaine Way (Western Health and Social Care Trust): The greatest delay during the six years was between the invitation for expressions of interest and the finalisation of the exemplar design. The Audit Office report states that the exemplar design procedure is a good approach to take; indeed, it has been adopted by Her Majesty’s Treasury. The process with the exemplar design involved analysing what would be best for the trust as the client, it set out a range of options, and it attempted to offer a design concept to the bidders. It was quite an extended process, but I believe that it achieved a better result, because we were engaging with our staff-side organisations and with the people who actually provide the services, some of whom you met today. By the time that we had finalised the exemplar design, a much better product was delivered.
20. The delay was not all down to the exemplar-design development; issues that concerned how the scheme would be procured had to be resolved. However, I draw the Committee’s attention to the development of an exemplar design, because in this case it was of huge benefit to the outcome.
21. The Chairperson: Are there any ways in which the project could have been completed in less than the six-year time frame with the same results?
22. Dr McCormick: If the project were commencing today, I would hope so. However, had we to go through the same process today, knowing only what was known then, I am not sure that it could have been completed any quicker, because it was breaking new ground and was untested territory.
23. Dr Jim Livingstone (Department of Health, Social Services and Public Safety): If one looks at previous PFI projects, the criticism generally was that they were taking too long, the negotiation procedure was sometimes difficult to control, and people were rushing in to the project. The problem was that, at the negotiation stage, it would often become clear that the bidder had misunderstood exactly what was required of them upfront. Those misunderstandings took more time to rectify, and they cost more money. The bidders were spending more time trying to understand what the authority wanted, and the more time that they spent, the more it cost. They then factored that extra cost into the overall price. There was a lack of preparation and clarity upfront, and that was adding to the cost.
24. Introducing the exemplar-design procedure required time and preparation, but that paid dividends later. It added time upfront, but it saved money later on. That is why the Treasury has adopted the procedure, which was developed in Northern Ireland’s Health Estates Agency. Although the procedure added time overall, it created a greater degree of certainty and greater clarity, and it saved money.
25. The Chairperson: Members will now have an opportunity to ask questions — each member will have approximately 10 minutes, and, although we require full answers, perhaps they could be as brief as possible.
26. Ms Purvis: Thank you for giving us a tour of the facilities this morning. I was extremely impressed with both the facilities and the dedication of the staff. The tour brought home to me the joined-up nature of services and delivering care for patients. In our work, we, as Members, do not often see the concern that staff have for patients. For example, a breast cancer clinic was being held today, and the staff were working very hard to deliver services to patients, which was tremendous. I therefore pay tribute to the staff.
27. Dr McCormick, my question relates to paragraphs 1.4 and 1.6 of the Audit Office report. One of the key arguments for using PFI is that it transfers risk to those who are best placed to manage it. However, in this case, the public sector took on the responsibility for the procurement of equipment, which will most likely develop and change over the next 25 years. I understand that the Centre for Cancer Research and Cell Biology in Belfast took the opposite approach, so why did you decide to opt for public procurement of equipment?
28. Dr McCormick: That was tested carefully in the procurement process. As the report shows, the negotiated procedure allows for variant bids, and a decision was made to allow core bids and variant bids in this project to test whether it was advantageous to include the equipment with the building in a single PFI contract. That meant that the procedure could be tested by actual bids from the proposers. Each case is assessed on its merits, and we have to address whether it represents value for money and whether it is affordable, in addition to asking what is advantageous from a client perspective.
29. As the evidence shows, the decision can go either way — a PFI contract was deemed to be the best approach in this case. The analysis of the bids showed that what was on offer was not as advantageous as using conventional procurement to get the required equipment.
30. Ms Purvis: You said that the equipment selection group that scrutinised the proposals identified major concerns. Will you elaborate on those concerns?
31. Mrs Way: A PFI contract was not used for the procurement of equipment because the clinical staff assessed the equipment that the two bidding consortia proposed as not being up to standard. It was stated in the evaluation process that using the equipment proposed by the consortia in our haematology laboratories would have made our service unsafe.
32. The trust employed six independent clinical assessors, as well as their own clinical staff, to examine the equipment. Those assessors raised concerns about the quality of the equipment that the consortia were proposing to provide and about the proposed timescale for refreshing the equipment. That was evaluated carefully, and it was judged that it would be best to take the equipment out of the PFI contract.
33. Ms Purvis: Mrs Way, as indicated in paragraph 1.4, HM Treasury research shows that procurement times are often disproportionately large for smaller PFI schemes such as this. Given those concerns and the length of time that the process for the centre took, how confident are you that value for money has been delivered in the long-term overall redevelopment of the Altnagelvin Hospital?
34. Mrs Way: I am confident that we have delivered value for money. The Audit Office report demonstrates strongly how critical it was that the laboratories and pharmacy centre proceeded at the time that they did.
35. The Department indicated that it was likely that public funding were to become available and that that would happen as late as this financial year, which is 2008-09. It is critical that the sequencing, which Alan Moore discussed with the Committee this morning, happened in that way. Any delay would have had a negative impact on the overall strategic redevelopment and on patient care. Altnagelvin Hospital could not have continued with the quality of laboratory and pharmacy services accommodation that it had. That is an important point.
36. We kept the public-sector comparator in the modelling for this contract right up to the last moment in order to demonstrate that the project was providing value for money. We were left in a position in which we had one shortlisted contractor and no reserve, so we used the public-sector comparator as a negotiating tool to ensure that we got value for money.
37. Ms Purvis: Does that mean that the other phases would have been held up?
38. Mrs Way: Yes, very much so.
39. Ms Purvis: Would the costs also have crept up?
40. Mrs Way: Yes, they would have increased.
41. Ms Purvis: Paragraph 1.4 of the report states that today, capital projects with a similar value would not be considered for PFI. Has the Department of Finance and Personnel issued any guidance to Northern Ireland Departments as to what is the appropriate level for PFI projects? Has it set a minimum threshold?
42. Mr David Thomson (Treasury Officer of Accounts): The Treasury has reviewed PFI projects, and its current guidance, which has been circulated, suggests that anything under £20 million is not appropriate for a PFI contract.
43. Mr Wells: Paragraphs 2.2 and 2.3 of the report examine the projections of the demand for the services that are provided by the new laboratory. Do you have any more up-to-date information on how demand is going? Have you reached the target or are you exceeding targets? What do you expect that the demand for the various services will be in the future?
44. I agree with Ms Purvis in that I was extraordinarily impressed with what I saw this morning. Could I have two such centres for South Down, please? I would like to be sitting here in 10 years with the Public Accounts Committee criticising South Down for having two centres such as this — this one is state of the art. Anyone who was on the visit this morning could not have been more impressed by what they saw. How accurate were the projections for demand?
45. Dr McCormick: The short answer is that the projections have been borne out. The level of need for the facility and the demand for its services, which were built into the business case, are broadly in line with expectation. The fundamentals relate to the nature of population growth in the area that is served by Altnagelvin. Those are basically in line with population projections. The elderly population has grown by 14% between 2001 and 2007, and that again is one of the major drags on demand for health and social care and hence for the services that are offered by this facility.
46. There was a response to the projected and identified real need of trends in population and health and in some of the aspects of pharmacology — such as dealing with cancer and heart disease — that were demonstrated clearly on the tour this morning. The trends are broadly in line with what was built in to the plans.
47. Mr Wells: Do you feel that the expenditure is justified on the basis of the throughput that we now see?
48. Dr McCormick: Yes, very much so. The laboratory is working as expected. There are obviously lots of peaks and troughs, and the flexibility to manage in real time and the ability to cope with what is a very demanding role as a subregional centre are required. Altnagelvin is the largest acute facility outside Belfast. It serves a large population and is now the main hospital for the Western Trust. The demand and the need for the services that are provided are clear, and they therefore justify the project’s proceeding.
49. Mr Wells: When the Committee considered the issue previously in 2002, it found that only 59% of pathology services in Northern Ireland were accredited. What is the percentage today, and when can we expect it to reach the desired 100%?
50. Dr McCormick: Some 79% of laboratories have either full or conditional accreditation. The Department’s recommendations for the future of pathology services, on which the Minister made an announcement in December 2007, commits us to achieving full accreditation by 2010. Since April 2008, all labs are required to be registered with the Clinical Pathology Accreditation (UK) Ltd, which undertakes the accreditation process independently. It is a rigorous process, and the commitment is to work towards achieving full accreditation by 2010. That will be challenging, because accreditation includes not only aspects of the physical facilities — the equipment in the labs — but it relates to staffing, which is not always easy to control. Planning and managing an expert workforce and ensuring the correct level of supply and demand through recruitment and retention is at times challenging, but accreditation depends on securing those elements.
51. The process is rigorous, but we must go through it to achieve full accreditation. We must also ensure that we have safe and effective services. If there is a shortfall against the ideal as set by the standard-setting body, we must ensure that we have safe and effective laboratory services throughout Northern Ireland — that is a vital part of the process and the reason that change and reconfiguration are necessary, as the Committee recommended in 2001.
52. Mr Wells: Out of interest, is the list of accredited labs available to the public, or does the Department retain that information?
53. Dr McCormick: I am sure that if you were to ask for the information, there would not be a problem in getting it.
54. The Chairperson: Perhaps we could have that information in writing.
55. Mr Lunn: I apologise for being unable to participate in the tour, but I am told that the facility is beyond impressive — I am not sure how best to describe it, but I believe that it is totally fantastic. If you managed to satisfy Jim Wells, it must be really something.
56. Mr Wells: That is because I want a similar facility for Daisy Hill.
57. Mr Lunn: He wants to take the robot home with him, apparently.
58. Although the facility is well placed to manage any increased demand for pathology services in the foreseeable future, paragraph 2.6 of the Audit Office report states that recruiting consultant staff represents a challenge. Have all those posts now been filled? Are your current staffing levels adequate to meet the expected increases in demand?
59. Mrs Way: At the moment, three of the five labs in Altnagelvin have full accreditation. Haematology and microbiology have achieved partial accreditation, but as you have been able to tell, that has nothing to do with the fabric of the building. The sole issue that has led to the conditional accreditation is the fact that, when the Audit Office report was published, we were short of two consultants in haematology and one in microbiology. Today, we are short of one consultant in haematology and one in microbiology. Members who went on the tour will have heard Dr Gerard Glynn confirm that we will interview candidates for that post in October, and we are hopeful of making an appointment. I should point out that we always work hard to fill vacancies. If there is a vacancy, we seek to fill it straight away.
60. I understand that there is a national shortage of pathologists — there are 59 vacant posts across the UK. A few weeks ago, I spoke to someone who was applying for a post in our labs, and they said that they were so impressed by both the working environment and the team that they decided that this was the place where they wanted to work. Hearing such remarks from an applicant confirmed for me that the quality of the building and the opportunities that it offers — including research opportunities — have improved our ability to recruit staff. I am therefore confident that we will be able to fill our vacancies.
61. Mr Lunn: Recruitment seems to have fallen back slightly: the report says that the trust was short just one consultant microbiologist at the time of the previous review, but now you have lost another one. Has somebody retired?
62. Mrs Way: No; there are three haematology posts, and at the time of the Audit Office review, one person was in post, and there were two vacancies. Currently, two of the three posts have been filled. There is also a locum, so, in fact, those posts have been filled.
63. We are funded for two microbiology posts. We had one post filled, and we have interviewed twice. On both occasions, successful applicants were appointed, but they took up posts in Belfast. That is always an issue for those of us who work in the west, which is why it is so important that we overcome some of the hygiene factors that can be a challenge, such as the fabric of buildings.
64. Mr Lunn: Dr McCormick, what is the overall position in filling consultant posts in the rest of Northern Ireland?
65. Dr McCormick: I do not have details of numbers of staffing levels. As regards the wider matter of accreditation, which would act as a proxy for that, some issues have arisen in other trusts. However, from the information that I have in front of me, I cannot distinguish whether that is to do with staffing or other issues. Perhaps I can provide some details later to draw out any hot spots or difficult areas in the recruitment and retention of specialist staff. Elaine has already pointed out that, with some specialities, availability is a UK-wide issue. Perhaps I could provide some details on that later, if that is OK.
66. Mr Lunn: Without going into detail, is filling posts a major problem across Northern Ireland at the moment?
67. Dr McCormick: It is an issue, but I would not describe it as a major problem. The majority of labs can secure essential staff. As is the case here, there are some vacancies, but it is not a critical problem.
68. Mr Lunn: How does that have an impact on service levels? What can you do to ensure that staffing levels are sufficient to meet future needs?
69. Dr McCormick: We are sure that we are delivering a safe and effective level of service across Northern Ireland. It is essential that that is the case. The Department has a responsibility to undertake workforce planning to ensure the right flow of training through the institutions and also to make sure that that is matched against projected levels of retirement, and so on. We must end up with the right balance, and we take the issue very seriously. As Dermot Hughes said during the tour, a large proportion of the expenditure in a facility such as this is on staffing. Indeed, he mentioned that staffing accounts for 66% of costs. Given that the costs are even higher across the entire health and social care system, a major part of our work involves effective planning to secure expert staff for all specialities in the future. We work very closely with the universities and other training institutions to ensure that that is the case.
70. Mr Lunn: I want to return to Jim Wells’s point about accreditation. Is it correct that full accreditation cannot be achieved without a full complement of consulting staff?
71. Dr McCormick: Yes, that is the case for full accreditation. As I said, the standard is high. Absence of full accreditation does not raise any issue about safety or the lack of safe services. It is a high standard that obviously must be met. However, the absence of accreditation is not an alarm bell or a danger signal.
72. Mr Lunn: That does not mean that there are certain tasks that cannot be carried out.
73. Dr McCormick: Tasks are still being carried out.
74. Mr McLaughlin: I agree with the comments that other members made — we had an impressive presentation and tour of the facilities this morning.
75. Paragraph 2.10 of the report states that two years after the major addition that this facility represents to pathology services began, you embarked on a review of regional needs. Is that the best way to plan for regional services? Would it not have been better to have carried out the strategic review before developing a regional facility such as this?
76. Dr McCormick: Ideally, yes. We had a range of wider issues about the provision of pathology services across Northern Ireland that had to be considered. Important recommendations had been made in the PAC’s 2002 report on pathology laboratories in Northern Ireland, and we had the major task of following through on that. The issue for Altnagelvin was simplified by the fact that around the same time the Developing Better Services strategy confirmed that Altnagelvin was to be one of the major hospitals for the future. Around 2002 to 2003, all doubt about the need for a full range of pathology services here was removed: we knew that those services would be needed. Many aspects of the detail of the configuration in other parts of the region were still being considered and analysed in the review, which was announced in 2003 and completed, with the Minister’s announcement, in 2007.
77. The project was developed in parallel with the work of the review, and those working on the project were aware of what was emerging from that review. Therefore, they were able to work in parallel to ensure that when it was necessary to make a decision about the project — and as they were committing to something that would last for 25 years — it would be possible to check whether it was in line with emerging thinking. The trend and nature of what was emerging was clear, even though the region-wide review had not reached a final decision. It was a managed process, and there was no conflict. It was not feasible to separate the processes, nor was it feasible to rush the pathology review, which was quite controversial in several areas and, indeed, remains so. We could not rush the review, nor would it have been right to delay the project until it had been completed. Neither of those options was available. The option of parallel running was the only available option, and we had to make it work. Therefore, the development of the project and the pathology review worked in parallel, and the two were informing each other.
78. Mrs Way: I commend the Department for the way in which it reviewed pathology services. Often, those of us who work on the front line believe that strategies are created in Belfast and those ideas are then imposed on us. However, on this occasion, the Department involved fully those people who were working on the front line delivering pathology services. I believe that that is the reason that the project took a bit longer.
79. As regards what Dr McCormick said about working in parallel, Altnagelvin Hospital was represented on the steering group of the review of pathology, headed by Dr Maurice O’Kane, who was also feeding in to the work that was being developed on the new laboratory and pharmacy centre. We understood the trends, and the team took that into full account.
80. Mr McLaughlin: I am hearing the message, but I need further reassurance. Paragraph 2.10 states that the 2006 review envisaged that the plans for the new laboratories: “should go ahead in such a way that the planned laboratory for histopathology and cytopathology can be used for other purposes when the new Belfast laboratory is operational.”
What does that mean? We have heard already about the dedication and commitment of the professionals working in the centre, but we have also heard about the difficulty of recruiting and retaining staff.
81. Paragraphs 2.2 and 2.3 of the report describe clearly the increasing need for pharmacy and laboratory services. Is there any shadow of uncertainty about the functionality of and the commitment to those services?
82. Dr McCormick: No, I reassure the Committee that there are no doubts about that commitment. During one phase of the pathology review, the possibility of centralising some aspects of pathology to Belfast was considered. Hence, that is the explanation underlying the point made —
83. Mr McLaughlin: I have read the Department’s response to the consultation.
84. Dr McCormick: The review concluded that Altnagelvin needed such a facility, and, therefore, the project was able to proceed. As we heard on this morning’s tour, there are rising demands and a need for flexibility and future-proofing in the facility. Therefore, there was no material risk of the team making the wrong decision and ending up with an over-specified facility. We kept in touch, and the possibility of centralisation was considered but was, ultimately, not favoured. Does that help?
85. Mr McLaughlin: Yes. However, I will poke my finger in the wound in your side a little further. [Laughter.] Given possible developments in Belfast, what are the long-term plans for the centre?
86. Dr McCormick: We are committed to providing, for the foreseeable future, the range of services that you have seen on this site. We signed a 25-year contract for the building. Medicine will evolve, and new equipment will, undoubtedly, materialise. If we take the trend in computing as an example, equipment will perhaps become smaller, and it is possible that less space will be required. On the other hand, innovations might require new procedures. No one can foresee technological advances. We have used the best information that is available and incorporated a sufficient degree of flexibility. In 10 years, something will, undoubtedly, have changed, but we have made the best decision. If we waited for certainty, we would achieve nothing.
87. Mrs Way: You will not be surprised to hear me confirm that I believe that the Department has made a clear commitment to the laboratories and pharmacy centre. The regional review of pathology was undertaken when there were — as Dr McCormick said — 18 trusts. The RPA has reduced that figure to five, and the Audit Office report and the regional review of pathology outlines clearly that the new structure, under the RPA, will allow the bigger laboratories to continue their work.
88. The Altnagelvin laboratory now has a much stronger leadership role in the new Western Trust. When I was chief executive in Altnagelvin, my chief executive colleague in the former Sperrin Lakeland Trust tried to sort out its discrete laboratory issues. The RPA has emphasised the importance of Altnagelvin’s laboratory. We are located in the north-west, and that location provides networking opportunities both in Northern Ireland and across the border. Members will have heard some of our clinicians talk about future-proofing by providing additional capacity for the laboratories. There is potential for that to happen, particularly as the Minister announced recently the establishment of a satellite radiotherapy centre here that will underpin the need for excellent diagnostic facilities on the site.
89. Mr McLaughlin: To what extent do you currently provide cross-border services to Donegal and the border counties?
90. Mrs Way: Those services are changing. Until quite recently, we provided significant cytology services for the Health Service Executive (HSE) to cover Counties Donegal, Sligo and Galway. The HSE advertised a competitive exercise for people to provide such a service, and I understand that the contract for that has just been awarded to an American company. However, the potential for the satellite radiotherapy centre to provide a cross-border facility will create the need for diagnostic facilities that will have to be funded by the HSE. I emphasise that no services are provided in the hospital unless they are funded fully by our colleagues from across the border. Therefore, the services are provided on the basis of a service-level agreement, but it is a bit of changing feast.
91. Mr McLaughlin: Has that fluctuation affected the business case for the centre or the review conclusions in any way?
92. Mrs Way: No, the potential for cross-border working was reflected in the business case, but given that it is changeable, it did not have a heavy weight.
93. Mr Beggs: I apologise that I was unable to take part in the tour of the building; I was at a meeting of the Committee for the Environment, which I left early to attend this meeting. However, I have been impressed by my colleagues’ comments about the building and the staff.
94. Mr Thomson, paragraph 3.2 of the report discusses exemplar design, which Her Majesty’s Treasury has recognised as being best practice. Has such an approach been used in other major capital projects in Northern Ireland in recent years?
95. Mr Thomson: DFP does not promote exemplar design as such. We have promoted an initiative called Achieving Excellence in Construction, which the procurement board agreed in 2005. I reassure the Committee that design is an integral part of that initiative. Under the initiative, design is developed by an integrated project team, which includes designers, the client and contractors. The traditional approach of seeing design as separate is no longer taken.
96. Outside the Health Service, exemplar design has not been used extensively, but it is used under the construction initiative. For example, the Belfast: Streets Ahead project was developed following the exemplar design that was commissioned by the Department for Social Development.
97. Mrs Way: Exemplar design worked well for us, and we have used the same exemplar-design approach for the south-west hospital to the north of Enniskillen.
98. Mr Beggs: To follow up to the Treasury Officer of Account’s answer, it appears that trusts have the flexibility to use exemplar design. Is there any guidance to say when it should not be used so that best practice can be exchanged? For example, a positive result has been achieved in the design of this building.
99. Mr Thomson: The procurement board, which looks after procurement policy in Northern Ireland, has not issued any particular guidance on that. Dr McCormick may wish to comment on the use of exemplar design in the Health Service.
100. Dr McCormick: As Elaine mentioned, exemplar design has been used more fully in the Health Service on the two major hospital projects in the Western Trust area at the Tyrone and Fermanagh Hospital and the work that is being done on the Omagh proposal. Elsewhere, aspects of phases of the Ulster Hospital have been worked on using that process. The exemplar-design process has also been used on a series of projects for primary-care centres, both in and outside Belfast. It is regarded as an accepted process. John Cole and his team in the Health Estates Agency are well used to using that process, and they are to be commended for having developed the idea.
101. Mr Beggs: Having been satisfied with positive outcomes, it would appear that the Department of Health and trusts have taken that on board. Are you aware of any other Departments that use the exemplar design process? In order to ensure that the people who will work in an area, and not just the architects, are kept in mind during a project’s design, it seems to make sense to listen to end users.
102. Mr Thomson: The Achieving Excellence in Construction initiative incorporates design — it is an integral part — and the initiative states upfront that design should be integrated into the whole construction process and that clients, users and the contractor should be involved. The initiative does not rule out exemplar design, but it does not state that one must incorporate exemplar design before going to the marketplace. That is the marginal difference. Therefore, the Achieving Excellence in Construction initiative allows one to have that dialogue.
103. Mr Beggs: Paragraph 3.3 of the report states that:
“the exemplar design was largely adopted by the PFI bidder with only very marginal changes.”
However, during negotiations with the bidder, changes to the exemplar design process resulted in significant cost increases, including the annual unitary charge. It is common knowledge in the construction trade — and most people are aware of this — that builders and developers bid using tight margins and subsequently make their profits on changes. Is this an example of going in with tight margins in order to profit from changes?
104. Furthermore, given the level of the negotiated increase, as set out in figure 5 on page 23 of the report, did the exemplar design process bring increased certainty to the project’s budget?
105. Dr McCormick: It is important to set that in the context of the stages of the process. Although I am familiar with the pattern of cost escalation that you mentioned, that did not happen in this project. The key fact to note is that the point at which the project’s budget was finalised was on receipt of the final business case in February 2005. Up to that stage, in addition to the issue with procurement during the negotiation process that I explained earlier, genuine changes that were necessary to the client were made to standards and to aspects of the design. After the project was finalised, it was delivered within budget and ahead of schedule.
106. Concerning the matter of when the contract was closed, we were in a genuinely evolutionary phase, and some iteration was required to ensure that the right things were being done. Legitimate changes were made to improve the design. For example, the changes that Anne Friel mentioned this morning to security measures that would protect the pharmacy had to be made between the initial stage and the approval of the final business case. In addition, changes had to be made with regard to accreditation and to achieve the best management of certain costs.
107. Those factors explain the changes that are shown in figure 5. They were legitimate aspects of the planning process, and it would have been wrong not to have taken account of them. As Elaine Way said, we monitored the evolutionary process tightly, from what the contractor said the project would cost to what a public-sector comparator would have cost had we gone down that road. Those matters were on the table right up to the point at which the final business case was approved, and the basis for the decision was transparent and open.
108. After the final business case was approved, the contracts had been signed, and everything had moved on, the project was delivered within budget and on time. I am confident about that, and it is important that we recognise that cost escalation did not apply in this instance.
109. Mr G Robinson: I apologise for not being present this morning; unfortunately, I had a lot of constituency work.
110. I congratulate the chairperson and the directors of the Western Health and Social Services Trust for bringing this much-needed facility to the north-west. My constituents in Limavady and further afield very much need the facility, and I am sure that it will be well used. However, I have a few questions that I would like to ask.
111. Mr Shannon: Some hard questions are coming now. [Laughter.]
112. Mr G Robinson: My first question is for Dr McCormick. Paragraphs 3.7 to 3.10 of the report show that there was some confusion surrounding the funding of the project. In July 2002, the intention was to deliver the project using PFI, but by October 2003, a publicly funded solution was seen as the way to produce the best economic outcome. Four months later, a PFI solution was chosen. Why was there such uncertainty about the funding of the project? How can you be confident that the most economically advantageous solution was chosen?
113. Dr McCormick: That is a complex matter. Its complexity and novelty is the main explanation for the issues that you highlighted and that are outlined in the report. We are committed to ensuring that in making decisions on how to procure projects of this nature, our prime consideration must be to secure best value for money.
114. We also have to examine carefully what is affordable, given the budgetary rules under which we operate. In the context in which we were working between 2002 and 2004, we understand that an opportunity existed to instigate PFI projects that would be funded from the revenue budget. The unitary budget for such projects would score against the recurrent budget, not against the capital budget. That understanding of how things would work changed later on, but that was the situation at the point of decision. The PFI option provided an affordability advantage. The question that the Department asked consistently throughout that period was whether we were sure that we were doing the right thing, whether we were ensuring that value for money was secured and whether we understood the possible options.
115. If we look back to 2003, at the time of the letter that was referred to and at the budgets that we had at our disposal then, we were coming to the end of a sequence of financial planning. We did not have large amounts of money available for capital projects in the planned future years. Major capital projects, such as those at the Royal Victoria Hospital, the Ulster Hospital and in other parts of the region made significant demands on resources, but were not so suitable for PFI.
116. The Department was asking a reasonable and rational question. Subject to the overriding consideration of securing acceptable value for money, would it not have been advantageous, from the trust’s point of view, to examine a PFI option? That option was considered openly and transparently, and, as outlined in paragraphs 3.8 and 3.9 of the report, the conclusion based on the available evidence was that value for money would be secured through the PFI option and would have the significant advantage of allowing the project to begin earlier than scheduled.
117. As Elaine Way said, that mattered so much in the total planning of the hospital complex. It provided certainty of both timing and budgets. Having weighed all those things up, it was the right decision. As the report says, there was a point in time at which the trust and the Western Health and Social Services Board had examined a business case that showed that public funding was more advantageous, but that was subject to further scrutiny and was not a final business case. Further scrutiny was undertaken to examine the details of the business case, and as time moved on, there was a critical need to meet the timetable and deliver the building so that phase 3 could begin as soon as possible. That was an important consideration, hence the conclusion that was reached.
118. I do not think that people were confused or uncertain about the matter, which was complex. The difference in numbers was not significant either way — it was a judgement call. After taking into account all the factors and the evidence available, we made our decision.
119. Mr G Robinson: Mrs Way, after comparing the options that are detailed in paragraph 3.9, the trust at Altnagelvin concluded that the PFI option could be expected to reduce the length of time that it would take to complete the overall development programme for the complex by at least six to 12 months. On what evidence was that based? What was the original timetable, and is it still on track?
120. Mrs Way: The timetable is on track. Phase 3 of the development was started early. The report shows that the contractor delivered the new centre early, which meant that we were able to progress with phase 3. Therefore, that is very much on track. As the permanent secretary, Dr McCormick, said, the PFI solution represented the best value for money. I was not in Altnagelvin at the time, but I have studied all the documents carefully in preparation for this hearing, and I am certain that I would have made the same judgement as my predecessor. The single biggest issue that needed to be taken into account where business cases and funding were concerned was the impact that it would have on the overall strategic development. As the permanent secretary said, that provided certainty over funding, it allowed for overall development to continue, it reduced the real risks to our services, which you heard about this morning, it enabled us to meet quality standards, but most importantly, we have demonstrated that best value for money has been provided.
121. Mr Shannon: I apologise for not being present this morning; I was at a meeting of the Committee for Culture, Arts and Leisure here in the Maiden City.
122. If Jim Wells says that he wants that equipment used in hospitals in South Down, it must be good stuff. You should be encouraged by his comments.
123. My questions concern the equipment for the centre. Paragraphs 4.1 and 4.2 of the report set out the context for the rejection of the bidders’ proposals for the equipment for the centre. With respect, it seems ludicrous to have provided a list of equipment that was already in use and that was past its sell-by date rather than to put more effort into building a detailed specification for bidders of equipment requirements. There is something wrong about a process that cannot deliver for the demands of a modern service centre. Will you comment on that?
124. Dr McCormick: The team sought to secure the right specification of requirements. At an early stage of the process, the team examined the building and the equipment. A great deal of work was put into the invitation to negotiate, which, as I said earlier, is a vital part of the process. Given that it was the first time that that particular team had undertaken the process, a great deal of effort and time was put into the specification of requirements. I view the rejection of the bidders’ proposals as a missed opportunity on their part rather than as the result of problems on the client side.
125. As Elaine said earlier, we were disappointed that the bidders’ proposals were unacceptable in the end, hence the decision to exclude the equipment from the process. The specification was not unhelpful or ill-founded, but the bidders missed an opportunity.
126. Mr Shannon: As a result of that experience, have you tightened up the system to prevent such events from happening again?
127. Dr McCormick: Other cases exist where managed services have been procured successfully for local contracts; Jim will give more detail on that.
128. Dr Livingstone: Such systems work, but the key thing is that a PFI procurement involves not just one company, but a consortium made up of a facilities management company and a construction company, for example. The consortium must then choose a company that will provide the equipment. Therefore, four or five companies could become involved. It is simple — if the consortium chooses the wrong partner on that part of the bid, they will not come up trumps. In the case of the cancer centre, for example, bidders became involved with partners to provide the equipment, and they were better at that job. However, we provided them with very detailed specifications. In a sense, we try to attract the best-quality bidders, but we cannot make the consortium choose their partners.
129. Mr Shannon: Can you control the specifications?
130. Dr Livingstone: Absolutely.
131. Mr Shannon: Perhaps some of the bidders fell down in that area. The equipment cost the public purse £3·1 million. How can you assure us that the taxpayer got value for money? What are the ongoing maintenance arrangements and costs?
132. Dr McCormick: Elaine or Jim may be able to respond to the latter point, but, on the main point about securing value for money, conventional procurement methods were used, which involves ensuring that the correct protocols and controls for testing them are used. Again, in that context, we must ensure that we specify the correct things and use the correct competitive processes. It is not a 25-year contract or a 10-year contract for provision of service associated with equipment, as in a PFI procurement of equipment, but a conventional procurement of equipment still has all the standards and objectives that ensure good value for money for the public purse. Therefore, the business case that was made for all the major equipment, including the robot that members saw in the pharmacy this morning, will have gone through the proper analytical and approval process to ensure that value for money has been secured for the maintenance and monitoring of the equipment.
133. Mrs Way: The Department benchmarks how much our labs cost to run, so it would examine whether Altnagelvin is a significant outlier that costs much more than other facilities, but that is not the case. On the tour this morning, one of the members was very taken with the robot.
134. Mr Shannon: I think that he wanted the robot to drive him home.
135. Mr Wells: Wait until you hear how cheap it was.
136. Mrs Way: I told a member of the Committee that the robot cost £300,000, but when I spoke to the head of pharmacy, she told me that it cost £252,000, with 6% revenue running costs per annum. As the permanent secretary said, all that would have been approved formally. All our purchases are made through a centre of procurement excellence. In all our major purchases, we always ensure value for money.
137. Mr Dallat: I wondered for a while whether this was the Public Accounts Committee — we normally tear the backside out of projects.
138. Mr McLaughlin: You are not going to let us down, are you?
139. Mr Dallat: I am, because many people from the Coleraine area come to this hospital, and they have nothing but praise for it. I am pleased that I survived the journey from Stormont to here, but I experienced the statutory delay in Dungiven. My only criticism of this facility is the car parking, but perhaps designated parking is available.
140. Mr Shannon: Is that because you had to pay for it? I can lend you my ticket.
141. Mr Dallat: It is hard to live up to my reputation, but the 21% increase in the cost of the project was down to construction costs and unitary cost. Will you explain why those factors were not identified during the development of the exemplar design? I picked up some of the reasons in Dr McCormick’s previous answer — the process was being developed as it went along.
142. Dr McCormick: The exemplar design had to be improved and changed as the years passed to make sure that it was in line with what was then current practice and to make sure that it was up to date at each stage. We had to ensure, before we committed to the contract, that we were building to a 2005 specification and not to a 2003 specification. It was very important that those changes were made. That meant that there was a higher cost difference between the final business case and the earlier stages of the business case. The changes that were made were genuine and legitimate. They included aspects of improving future-proofing, security and ensuring proper life-cycle maintenance. They were not responses to unacceptable or undesirable pressures from the bidder — that was not what was happening. They were genuine client needs, and that is why they were built into the public-sector comparator.
143. We compared the PFI and public-sector routes at each stage, both in February 2004 and in February 2005. We asked which was best, and how we could pragmatically ensure the best outcome for the service.
144. Mr Dallat: On reading paragraph 4.5 of the report, it is encouraging to see the trust and its advisers being vigilant in protecting the interests of the public sector. I agree strongly with that. Will you explain how the issue of corporation tax was identified, what is the latest estimate of the potential savings figure, and why the agreement does not provide for 100% recovery from the contractor?
145. Mrs Way: The issue of corporation tax was identified by the trust and its advisers. I do not consider myself to be an expert on this, but my understanding is that as the process was progressing, the potential operator — the contractor — had included a contingency. That was to do with a potential corporation tax liability on any construction profits. The trust team, supported by its financial and legal advisers, identified that the operator had built in the fact that they believed that the liability would become payable upfront from the point of handover of the building. The trust and its advisers said that that would not be the case and that HMRC was not likely to take that view. They negotiated an agreement with the operator whereby if HMRC agreed to their interpretation, 50% of the share should be returned to the trust. At this point in time, the benefit to the trust is estimated at £130,000 as a one-off payment.
146. Mr Dallat: Paragraph 4.7 states that the additional advisory fees were due in part to initial advice on the nature of the implementation timescales being unrealistic. That paragraph also explains that some of the delays were outside your control. Will you explain to the Committee what factors were outside your control and who provided the initial advice that led to the incurring of additional fees? Indeed, if that advice was wrong, why should the public sector pay for it?
147. Dr McCormick: The starting point was that an estimate was made of the amount of fees to be paid. A budget was negotiated and secured and then approved by the Department. That budget was set in September 1998 at £150,000 for the consultancy. The original proposals were within that range. We now know that that figure was significantly lower than that which was incurred in the out-turn. That was because the project turned out to be significantly more complex than had been anticipated.
148. That was genuinely outside the control of the trust and the Department. The right approach was for us to proceed properly and to test at each stage what would be required from the next stage of consultancy advice. Initially, we had a fixed fee with no right to go beyond that amount. There was a provision to negotiate increases, step by step, as necessary. However, any increase always required further approval and negotiation with the advisers to ensure that we were not getting into any kind of open-ended commitment, which we did not do. It is worth emphasising that, in benchmark terms, the total fees were not excessive.
149. Mr Dallat: My final question really requires a written reply. When the PAC examined pathology services previously in November 2001, it was told that Altnagelvin had the highest prices for GP laboratory tests in 14 out of 32 tests. Can you bring the Committee up to date on the current situation? A written response would be fine.
150. The Chairperson: Are you happy enough to do that?
151. Dr McCormick: Yes.
152. Mr Craig: I went on the tour this morning and “impressed” is perhaps not the right word to describe how I felt — the little green giant came out in me. Like Jim Wells, I have been fighting hard to keep what few facilities are left in Lagan Valley Hospital, and I am darn jealous of what has been achieved here.
153. Mr Shannon: You should check his car on the way home. [Laughter.]
154. Mr Craig: You are right; there could be a robot in the boot. [Laughter.]
Having worked in robotics for the past seven years, I am probably one of the few people here who could dismantle the robot and take it with me, so beware.
155. Mr Shannon: Is this conversation being recorded?
156. Mr Craig: I am astonished that nobody has given the robot a name yet — that is usually the first thing that happens in a company.
157. We want to know whether the project was carried out properly and whether it was good value for money. Paragraph 4.9 of the report hints at the point that the project team has not evaluated its own internal costs for managing the project. We accept that the cost of advisers to this project compares favourably with other projects. However, has any attempt been made to evaluate the team’s internal costs, given that they were probably pretty extensive on a project of this scale?
158. Dr McCormick: Important work was done. I would not describe the team as extensive; quite a small number of people were involved, and they had a great deal of responsibility. They are to be commended for managing the project effectively and for seeing it through without excessive cost. We acknowledge that we did not introduce the recommended time-recording system. However, the truth is that the work was undertaken primarily by the people from whom you heard this morning and that not much other full-time in-house work was involved.
159. It was crucial for the core team to involve heavily clinicians and professional staff in order to ensure that what was being procured would provide the most effective service delivery. That was key to the process. The team had to work extensively with their colleagues in the trust to ensure that that happened so that both parts of the building were really effective. There was some input from heads of service — the estate services manager, the science services manager, and so on — and others, but the project was led by a small core team.
160. We recognise that there is value in more detailed time recording, but my instinct is that, if we had introduced a time-recording system, it would have been relatively modest and proportionate. When we consider the value of what was obtained, we must pay tribute to the way in which the trust handled the process.
161. Mrs Way: You are absolutely right. We should have taken account of the costs of the in-house team; we have also learned that we were over-reliant on a couple of experts in our organisation. I have certainly learned that lesson for the future. This morning, you saw Alan Moore and Paul Quigley, who were the main leads on the project. If either one of them had been knocked down by the proverbial bus, we would have found it difficult to replace them. David made the point that PFI would not normally be used for a project of that value, but a big project needs a properly costed project management structure.
162. Mr Craig: Dr McCormick, paragraph 4.9 says that the Comptroller and Auditor General, in a 2004 report, recommended that the costs should be monitored. Will the Department learn from those recommendations and implement them in future projects? Will it consider the costs of this project?
163. Dr McCormick: Yes, I accept that we need to do that. That was recommended before and has been highlighted again in the report. We need to act on that. It is not possible to re-examine the evidence from the most recent project, but we must accept your steer to take those recommendations on board for the future. We will act on that, especially if it is confirmed in the Committee’s report following this evidence session. We will ensure that we fall into line with that.
164. Mr Craig: I will note that, because one of my bugbears is that even though reports are produced, people continually ignore them. I will hold you to that assurance.
165. Dr McCormick: I take your point. It is not our intention to ignore reports, although we did not act on that one.
166. Mr Craig: Mrs Way, paragraph 4.10 states that you started a process of project evaluation in March 2008. Has that evaluation produced any findings?
167. Mrs Way: A draft post-project evaluation document is ready. It confirms that the business case was sound and that the project has provided value for money.
168. Mr Craig: Dr McCormick, the whole project was considered to be low risk, which meant that a full Office of Government Commerce Gateway review process did not have to be carried out. Considering that the project cost £250 million, would it have been better to carry out the full review process?
169. Dr McCormick: The capital cost of the building was £15 million, and a further £3 million —
170. Mr Craig: I am referring to the cost of the redevelopment of Altnagelvin Hospital as a whole.
171. Dr McCormick: An Office of Government Commerce Gateway review process would have been the appropriate action to take at the right stages. As the next phases of the redevelopment of Altnagelvin unfold, we must ensure that we complete processes in a structured and organised way in order to learn appropriate lessons.
172. Mrs Way: Although I have not been involved in an Office of Government Commerce Gateway review process, I took part in a health check, which was quite rigorous.
173. Mr Craig: You will be glad to know that that is the end of my questions.
174. The Chairperson: Mr Wells wants to ask about the cost of the robot.
175. Mr Wells: The robot is the least of my worries; it is remarkable value. Dr McCormick, imagine that you had walked into the permanent secretary’s office in DHSSPS on the first day with no knowledge of the project. If you had seen the project in operation, would you have had any qualms about signing the cheque for the final bill?
176. Dr McCormick: On the basis of the evidence I have now, I would have had no qualms.
177. Mr Wells: Have we reached the correct destination by a circuitous route?
178. Dr McCormick: The route was long. The team took the right decisions at the right time. It is difficult to know how the process could have been conducted differently. Some stages could, perhaps, have been shorter; for example, we could have resolved the choice between public- and private-sector routes more quickly. However, the process was managed effectively, and it allowed specific requirements to be secured and considered properly with correct clinical involvement and correct assessment of need. The fundamentals are sound.
179. Ms Purvis: Can the Committee have a copy of your post-project review when it has been completed?
180. Mrs Way: Absolutely.
181. The Chairperson: Do members have any further questions?
182. Mr McLaughlin: This question might apply in other circumstances. Internal costs were not evaluated. Dr McCormick might answer the question because it is not specific to this project. Is there any evidence that not evaluating internal costs affects the judgement between traditional public procurement methods and PFI?
183. Dr McCormick: Both methods place significant demands on internal teams; however, I am unsure how large a differentiator that is. There is a bigger difference in the extent and scale required for external advice, as both procedures require significant in-house resources —
184. Mr McLaughlin: Although this is not the most massive project, it is, nonetheless, surprising that that factor was not considered germane. Are the costs similar for both routes, or is there a difference? Is there a reason that that was not considered?
185. Dr McCormick: I am unsure whether there is a strong reason. I cannot see a major difference in the in-house team costs, because that will depend mainly on the complexity of the project and the detail that needs to be specified, all of which will be required in both routes.
186. Mr McLaughlin: PFI has its own persona, so to speak. The public and the private systems tend to look for problems and deficiencies. The value of the PAC’s work is to identify and rectify mistakes in order to close the circle. Is the idea of the virtuous circle in play here — adding value to the money that is available, for example, to a Department? That system has not always delivered value for money. It would be useful to know whether those costs are the same, irrespective of the selected route in order either to set them aside or give them ongoing consideration.
187. Dr McCormick: That is a fair question, which we will seriously consider.
188. Mr Beggs: PFI is meant to increase flexibility, innovation and design. In this instance, did your PFI partners bring additional thinking and originality to the final build to improve the complex?
189. Dr McCormick: They stuck closely to the exemplar design, and it was necessary to harmonise with the phase-three building. Therefore input was, perhaps, less than in some cases. However, there was some innovation and development.
190. It is worthwhile examining the case pragmatically and judging on the evidence.
191. Dr Livingstone: PFI contracts were promoted in the early 1990s, as it was claimed that they would increase innovation. However, in reality, there are two or three bidders in a very tense and competitive environment, and the problem is that innovation means risk. History shows that many bidders have not been prepared to take enough risk with innovation in case they do not win the competition. That has been disappointing.
192. Nevertheless, one of the benefits of the exemplar design is that bidders are shown an outline and have to say how they will improve on it; that offers opportunities for innovation. However, it is generally recognised that, in many cases — although not all — PFI has not delivered the innovation that we had hoped for.
193. Mr Thomson: One of the other major objectives of PFI contracts was the transfer of risk to the appropriate party. On the tour this morning, many people realised that although gaining agreement on a contract was a lengthy process, a very good building went up very fast. The risk was transferred completely to the PFI provider, which did not receive a penny until the building was up and operating.
194. An aspect of traditional procurement is the huge overrun in costs at the building stage, which continues afterwards; therefore transfer of risk is as much a consideration as innovation. PFI is not always the best option — most procurement is still done traditionally. Nevertheless, transfer of risk is an important element.
195. Mr Craig: From examples of PFI contracts that the Committee has examined, it seems that a lack of change control in a project causes costs to run out of control. Have there been many changes to the specification of the building throughout the project; or were all changes made up front so that the cost of the project did not change at the end?
196. Dr McCormick: After the final business case was agreed and the contract signed, the project ran as specified and was completed ahead of time, on budget and without changes.
197. Dr Livingstone: To its credit, the trust anticipated guidance that emerged to manage the contract on an ongoing basis — the trust has appointed a contract-monitoring officer, who is tasked with keeping a close eye on the contract to ensure that any changes that arise are well managed.
198. Mr G Robinson: It was nauseating to hear the comments about the project on one of the local radio stations. Visits like this verify everything that you have done and tried to do. I took great exception to those comments.
199. Mrs Way: I often say that, were it not for the Western Health and Social Care Trust, BBC Radio Foyle would close. [Laughter.] Recently, I asked a colleague from England how many times he is contacted by the media each month, and he answered three — I was contacted 106 times in the month before I spoke to him.
200. The Chairperson: Perhaps you will become a member of the Public Accounts Committee, Elaine, with comments like that. [Laughter.]
201. Mr McLaughlin: Or Radio Foyle.
202. The Chairperson: You have done a great job in getting the Public Accounts Committee out of Belfast for the first time.
203. There is a clear need for the improved laboratory and pharmacy facilities at the hospital. The high standards of the new facilities will enable the trust to meet the increasing demands in the new accreditation, benchmarking and clinical requirements. However, it took more than six years to complete the centre’s procurement, resulting in unitary costs increasing by almost £4 million over the 25 years of the contract. Initial uncertainty about the project’s funding method contributed to that. However, although there are benefits in using the exemplary design, it is imperative that it be applied in a timely way.
204. This was a relatively small project with regard to the scale of PFI. Nevertheless, the lessons will be examined in a report on this session.
205. The Committee expresses its thanks to the witnesses and to the staff.
Appendix 3
Correspondence
_fmt.jpeg)
Public Accounts Committee
Public Accounts Committee
Room 371
Parliament Buildings
BELFAST
BT4 3XX
Tel: (028) 9052 1208
Fax: (028) 9052 0366
Email: Jim.Beatty@niassembly.gov.uk
6 October 2008
Dr Andrew McCormick
Accounting Officer
Department of Health, Social Services & Public Safety
Room C5.11
Castle Buildings
Stormont
Belfast
BT4 3SQ
Dear Andrew
RE: Public Accounts Committee Evidence Session 2 October 2008
Further to the evidence session at the Public Accounts Committee yesterday, please provide the following additional information which members requested at the meeting:
1 Details of which laboratories are and are not accredited and consultant vacancies across the Western Trust.
2 The current costs of individual pathology procedures in the new Laboratory at Altnagelvin, relative to the costs of the same procedures at other laboratories across Northern Ireland.
3 A copy of the completed post project evaluation, when it is available.
4 Are internal costs the same, regardless of whether the route taken is Private Finance Initiative or conventional?
I would appreciate a response by Monday, 20 October 2008.
Yours sincerely
![]()
Paul Maskey
Chairperson
Public Accounts Committee
Letter to Paul Maskey - PAC Evidence Session 2nd October 2008


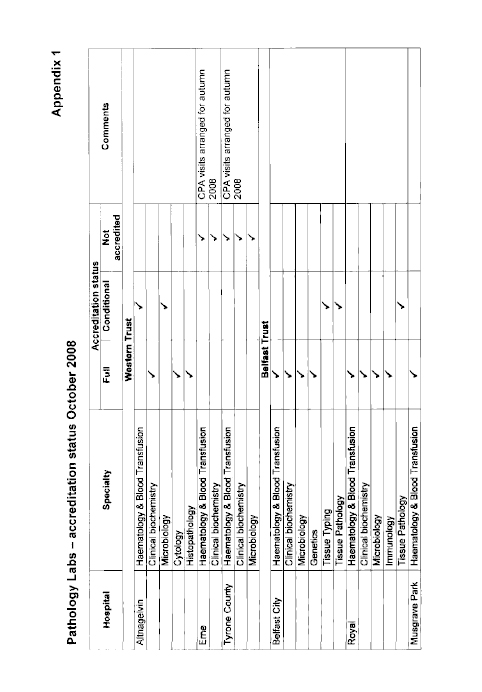
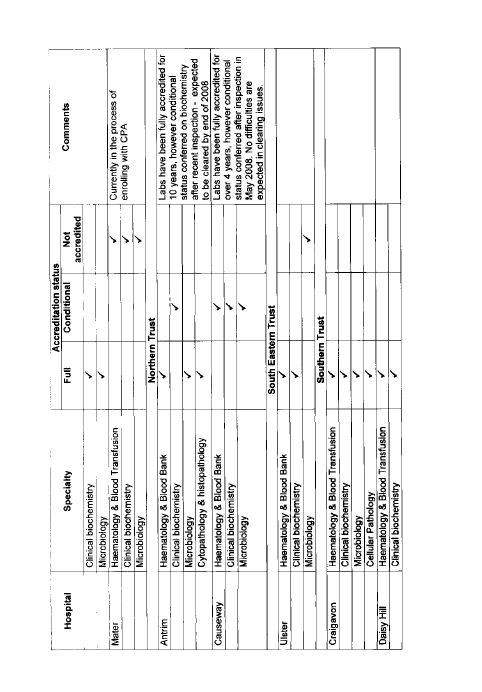
Letter to Paul Maskey - PAC Evidence Session 2.10

Appendix 4
List of Witnesses Who Gave Oral Evidence to the Committee
1. Mr Andrew McCormick, Accounting Officer, Department of Health, Social Services and Public Safety.
2. Mrs Elaine Way, Chief Executive, Western Health and Social Care Trust.
3. Dr Jim Livingstone, Director of Safety, Quality and Standards, Department of Health, Social Services and Public Safety.
4. Mr John Dowdall CB, Comptroller and Auditor General, Northern Ireland Audit Office.
5. Mr David Thomson, Treasury Officer of Accounts, Department of Finance and Personnel.
